In the pursuit of mass production of electronic parts necessary for our modern technological way of life all over the world, we have amassed a large amount of electronic waste(E-waste) which can be defined as unwanted used electronic devices/parts and the refuse they produce. These large dumps of waste not only take up massive amounts of space and can potentially become toxic but they also have large amounts of valuable materials like copper, cobalt, and gold that are stuck attached to thrown away reusable parts. Thankfully there are processes by which it is possible to remove said materials from E-waste and save the recyclable materials through chemical means: some call it "ubran mining". In an article from ETH Zurich, they explain a new non-toxic process by which they are able to recover gold from E-waste.
The process by which they recovered gold was by using a sponge made of denatured whey protein matrixes. Said proteins would form together into nano-fibrils in a gel structure. After drying the structure becomes a sponge capable of selectively absorbing gold from ionized metals. After absorbing the gold ions the sponge can be heated until the gold forms into flakes and can be removed and reused for new purposes. Their test resulted in a 91% gold nugget with the other 9% being mostly copper.
Another example of chemically stripping precious metals from E-waste is hydrometallurgy or using liquid-based chemical reactions to remove said metals. EU researchers were able to break down computer parts into centimeters worth of pieces, they then put them in an acid solution to break down the metals and then subjected them to chemical reduction(ascorbic acid for E-waste). This method, which was tried with different acids, was also particularly effective with it leaching 99% of the gold, 89% of platinum, and 100% palladium.
In the end, it is possible for governments around the world to partially reduce the amount of E-waste, pollution, and wasted resources produced by the ever-churning need for more technology by simply pushing more for these sorts of methods of material recovery. The protein sponge method is even made to be more cost-effective than simply dumping or using pyrometallurgical methods(which is also less eco-friendly). However, while better options are always being studied and tested massive change will be slow with our current system so all we can do is spread awareness and advocate for these changes
References:
- Bergamin, Fabio. “Turning Waste into Gold.” ETH Zurich, ethz.ch/en/news-and-events/eth-news/news/2024/03/turning-waste-into-gold.html. Accessed 12 Mar. 2024.
-N/a. “Electronic Hazardous Waste (E-Waste).” Dtsc.ca.Gov, dtsc.ca.gov/electronic-hazardous-waste/. Accessed 12 Mar. 2024.
- N/a, Directorate-General for Environment. “E-Waste: Chemical Processing Without Heat May Offer Efficient Method of Recovering Metals from End-of-Life Products.” Environment, 12 Oct. 2022, environment.ec.europa.eu/news/e-waste-chemical-processing-without-heat-may-offer-efficient-method-recovering-metals-end-life-2022-10-12_en#:~:text=The%20process%20involves%20leaching%20elements,central%20processing%20units%20(CPUs).
Posted for Antonio Kamin


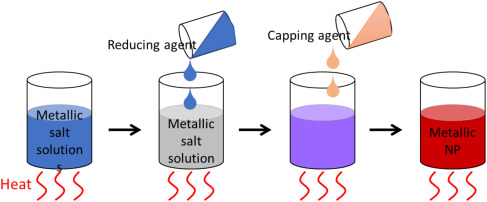
This deals with an important and interesting problem. Your title is fine but something with gold it ("mining gold from e-waste" or something) it might be more likely to draw in viewers. The graphics are good. They are eye-catching a visualize the result and the process that are the subject of the article. The figures might benefit from captions, however. Your lede (first) paragraph quite effectively outlines the problem. Your description of the process involving the whey protein sponge would benefit from a little more detail on how the sponge fits into the process. Metal containing parts of a circuit board are placed in an acid bath that dissolves and ionizes the metals. It is from this solution that the sponge selectivey absorbs the gold. Your source article is from a somewhat obscure source (at least here), but it deals with an interesting and important problem. It also highlights the possible role of chemistry in solving the problem.
ReplyDelete