Climate change has been one of the most talked about topics recently. The most asked questions regarding this topic include what the general population can do to help? And are we too late to reverse the damages? We live in a society where majority of people around the world do certain things that damage the environment. Carbon dioxide is the main chemical that further worsens climate change. Recently, scientists have been looking for solutions to minimize the amount of carbon dioxide that is released into the atmosphere. These scientists look at the reactions for carbon dioxide hydrogenation. Stated in Science Daily, various catalysts are currently used for carbon dioxide conversion reactions, most of them were designed and investigated for use in high-temperature and pressure conditions. These conversion reactions have many limitations which make it more difficult to find the solution to minimize the release of carbon dioxide. The limitations include expensive systems, the carbon dioxide hydrogenation favors lower temperatures, and high temperatures can affect the stability of the catalyst.
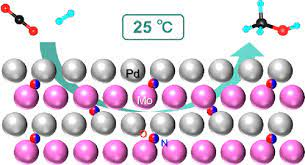
Figure 2: h-PdMo at Room Temperature
Professor Hideo Hosono from Tokyo Institute of Technology, Japan developed a better catalyst for carbon dioxide dehydrogenation. They developed an inter-metallic catalyst synthesized by a simple ammonolysis process by combining palladium and molybdenum. The scientists used an oxide precursor in the ammonolysis. During that process metals are combined by a mixing precursor, with ammonia gas at high temperatures. In Professor Hideo Hosono’s Journal of the American Chemistry Society article, found that p-PdMo was stable at temperatures up to 400 degrees Celsius and did not decompose in air.
Figure 3: Rate of h-PdMo at Varying Temperatures and Pressures
The results of this experiment found that h-PdMo for carbon dioxide dehydrogenation was capable of continuous methanol production at room temperature and low pressure. This is huge because carbon dioxide hydrogenation reactions usually required a catalyst at high temperature and with relatively low pressure. This is huge with being able to convert carbon dioxide into methanol. This can help reduce the amount of carbon dioxide that gets released into the atmosphere. Therefore, help slow down climate change.
References:
Room-temperature CO2 hydrogenation to methanol over air-stable HCP-PdMo ... (n.d.). Retrieved April 17, 2023, from https://pubs.acs.org/doi/10.1021/jacs.2c13801
ScienceDaily. (2023, April 5). Opening a new frontier: Pdmo intermetallic catalyst for promoting CO2 Utilization. ScienceDaily. Retrieved April 17, 2023, from https://www.sciencedaily.com/releases/2023/04/230405112118.htm


I think "The Key to Slowing Climate Change" would be better for the second part of your title which is otherwise effective. The first picture is effective emphasizing that the fate of the climate is in our hands. Your lede paragraph takes a while to get to the main point: workers have found a way to efficiently convert carbon dioxide into methanol. You do make the important supporting points that carbon dioxide is the most important greenhouse gas and that greenhouse gases are heating the planet. Science Daily is a step up from the primary literature but is not as widely read as many more general interest sources. The more detailed description of the chemistry is quite good. The cartoon of the catalytic process is effective. The quantitative graphics make their point but might be require some effort on the part of the general reader. You say dehydrogenation in a couple of places where you mean hydrogenation. Something worth mentioning in a post like this is that the new process would help with climate change only when combined with a green source of hydrogen (like electrolysis of water using some carbon free electricity source). I think the piece adds to a positive image of chemists and chemistry as a problem solving resource. Overall a timely and interesting post.
ReplyDelete