A blog authored by "Chemistry in the Media", a class at the University of Delaware, dedicated to exploring and breaking stereotypes and stigmas applied to science and scientists by the media.
Tuesday, November 11, 2014
A Clear Way to Obtain Solar Energy
The world is constantly looking for cleaner ways to produce energy, and now there is a way that is barely noticeable to the average person. A team of researchers at Michigan State University have created a transparent luminescent solar concentrator that can be used on buildings, cell phones, and any other device that has a clear surface. This article explains what this new technology is made of and how it is used to harness solar energy. The transparent luminescent solar concentrator uses small organic molecules developed by the research team on a plastic-like material that absorb specific non-visible wavelengths of sunlight. The materials pick up the ultraviolet and the near infrared wavelengths that “glow” at another wavelength. The glowing light is then guided to the end of the plastic where it is converted to electricity by thin strips of photovoltaic solar cells. Because these materials do not absorb or emit light in the visible spectrum, they look transparent and do not block the view. So far, the solar conversion efficiency is close to 1 percent, but the researchers aim to reach efficiencies beyond 5 percent when fully optimized. Although it is still in its early stage, this new solar concentrator can produce energy in a non-intrusive way and has the potential to be scaled for commercial or industrial applications. (Communicated by Erika Ritchie)
Monday, November 10, 2014
Untangling Unknown Structures in Mixtures
Scientists have come up with a way to determine the structures of individual components in an unknown crystalline powder mixture. By using two existing techniques together, the separate crystal structures can be determined. Powder X-ray Diffraction (PXRD) uses diffraction methods to determine a "fingerprint" for every crystalline structure. This works fine for pure powders, but when a mixture comes into play, the two diffraction patterns can merge making it difficult to determine the components. By pairing this with Band-Target Entropy Minimization (BTEM) in a new method known as the PXRD-BTEM-Rietveld method, it is now possible to pick apart the structures and characterize the unknown components in a powder mixture. This new method could potentially be used in pharmaceuticals by investigating polymorphism.
The article can be found here.
The article can be found here.
Wednesday, November 5, 2014
The First Enzymatic Fuel Cell That Can Convert Energy to Electricity at Room Temperature
Engineers from University of Utah have now developed the first room temperature enzymatic fuel cell that uses enzymes, alkane monooxygenase and alcohol oxidase, to help jet fuel convert energy into electricity. Converting traditional jet fuel cells into electricity is difficult "because jet fuel contains sulfur which can impair metal catalysts used to oxidize fuel”. The research team investigated Jet Propellant-8 or JP-8, and concluded that using enzymes as catalysts, JP-8 fuel cells can operate at room temperature and can tolerate sulfur. The report is here, and the research article can be found here.
How Tylenol Works
The American Chemical Society produces a video series called "Reactions: Everyday Chemistry," which can be easily viewed on Youtube. Each short video gives a brief explanation of different chemical phenomenon and informs the viewer of how chemical compounds affect our everyday lives. This video in particular is about how tylenol works in our bodies to eliminate pain. Three different theories on the mechanisms of acetaminophen in the body are presented in the video, but in the end it is discussed that these three theories may work in combination to produce the effects of the drug. This is interesting and relevant considering that most of us take drugs like tylenol, advil, aspirin, and aleve without any knowledge of how these chemicals work inside our bodies. I also thought that the comparison between synthetic pain killers and the natural pain killer THC in marijuana was relevant considering the fairly new laws legalizing this natural drug in certain states.
Thursday, October 30, 2014
Telomeres, Soda and Long Life?
The Washington Post picked up on a study linking consumption of sugared soda with DNA telomere length. The study claims that this link implies a link between sugared soda consumption and aging. The most dramatic claim as described by the WP is the following:
According to the research, drinking a 20 ounce bubbly beverage every day is linked to 4.6 years of additional aging. You get the same effect by smonking, said UCSF postdoctoral fellow Cindy Leung, lead author of the study. About 21 percent of the sample said they drank at least that much soday per day. However, researchers say, a link does not mean causation.Many other outlets picked up the story. Headlines included That Sweet Drink May Age You (CNN), Drinking Sugary Soda Makes Your Cells Age Faster, Study Suggests (Huffington Post), Perils of Drinking Sugary Soda: Weight Gain, Cavities, Shortened Lifespan and What? (Dallas News) and so on.
But are these claims justified. Most of the media simply reported the claims of the researchers as described in a post on the web site home page of their institution (UC San Francisco). In fact, the connection between telomere length and health is controversial as pointed out by Daniel Engbar at Slate in a piece headlined, Does Drinking Soda Really Age Your Cells? His answer to the headline is given in a sub-heading: How the Science of Telomeres Turned into a Spurious Health Trend. According to this Slate piece the basic science of telomeres is that they are strings of nucleic acid molecules at the ends of our genetic strands of DNA whose function is to keep the very long DNA strands from unraveling. These telomeres get shorter every time the cell divides until they disappear and the cell line dies.That much is uncontroversial. However, the connection between measured telomere lengths and any aspect of health or even aging is tenuous at best. Engbar's position is:
The shallow write-ups and inveigling headlines are insulting, and possibly injurious. In this case, though, they’re less offensive than the underlying science. The newly published paper delivers a mishmash of suspect stats and overbroad conclusions, marshaled to advance a theory that’s both unsupported by the data and somewhat at odds with existing research in the field. Its authors are less concerned with the health effects of drinking soda than with their broader project to establish a still new and fuzzy concept—“cellular aging”—as a pole star for public health.
Engbar also points out that the study authors stand to gain from book sales, speaking fees and product endorsements if they can persuade the public to accept their assertions about telomere lengths and health.
This is a good example of the extent to which media reports of science need to be considered carefully.
Monday, October 27, 2014
The Chemistry Behind Glow Sticks
Did you know that glow sticks were originated when a scientist tried to replace the natural bioluminescent light that fireflies made? This dates all the way back to around the 1960's when scientist Edwin Chandross, discovered through his experiments what chemical process was needed to occur in order for the glow stick to successfully illuminate. A lot of people are very much aware of what glow sticks are, but no one really understands the chemistry behind what makes it glow.
Inside the glow stick are two different chemical solutions, one of them being diphenyl oxalate, with a certain dye depending on what color you want the glow stick to glow. Then on the other side of the glow stick is the hydrogen peroxide. When one snaps the glow stick in the middle, the diphenyl oxalate is oxidized by the hydrogen peroxide which then produces the unstable compound (1,2-dioxetanedione). This is the reaction called chemiluminescence and when the reaction dies out, the glow stick will stop illuminating.
Quick Facts:
Inside the glow stick are two different chemical solutions, one of them being diphenyl oxalate, with a certain dye depending on what color you want the glow stick to glow. Then on the other side of the glow stick is the hydrogen peroxide. When one snaps the glow stick in the middle, the diphenyl oxalate is oxidized by the hydrogen peroxide which then produces the unstable compound (1,2-dioxetanedione). This is the reaction called chemiluminescence and when the reaction dies out, the glow stick will stop illuminating.
- To make your glow stick last longer, stick it in the fridge! Cooler temperatures means a slower reaction, hence a longer glow!
- Skin contact with these chemicals inside the glow stick can lead to irritation and dermatitis
Article Link Here
Lighting up the Brain
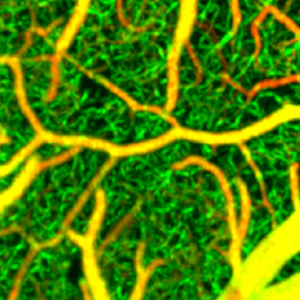
Imaging the brain has been notoriously difficult. Most methods require the subject to be dead, if not they are very inaccurate. This article discusses a new method of brain imaging that allows accurate images of brain tissue while the subject is alive and there is no need for a label, like a dye. There is a catch however, this method cannot penetrate the skull, so at the moment human application is limited to images during open brain surgery. However this opens up many exciting research options in animals. While this technology is still pretty new it has a lot of room for development. I thought it was pretty relevant considering that the Nobel Prize was given out this year due to advancements in spectroscopy and we just watched a movie about the development of staining. This technology can allow for non invasive imaging of a living brain. This can greatly aid advances in neuroscience, and the help unravel the mysteries of the brain.
Thursday, October 23, 2014
Series G Nerve Gases
First Human Trials of Stem Cell Therapies
Stem cell treatments have been used with a plethora of
diseases and conditions experimentally and in trial. Some of these include;
diabetes, Alzheimer’s, osteoarthritis, and spinal cord injury repair. Stem
cells are undifferentiated biological cells that can differentiate into
specialized cells and can divide to produce more stem cells. In a recent
article, a young woman paralyzed from a motor vehicle accident successfully
underwent the first in-human experimental procedure. The hope is that the
placed neural stem cells will bridge the gap created by the injury, replace
severed nerve connections and restore motor/sensory function. UC San Diego
Moores Cancer Center and the Sanford Cell Clinical Center (where the procedure
took place) are the leading research institutes when it comes to stem cells.
They have hopes for continued research and procedures with other disease such
as Type 1 diabetes and chronic lymphocytic leukemia.
Zaina Banihani
Sunday, October 19, 2014
Oxygen-Free Recycling Technique Could Keep Tons of Plastics from Landfills
Pyrolysis, the process of turning plastics into diesels and oils, is being used to keep non-recycled plastics from going straight to a landfill. A new analysis by the American Chemistry Council stated that a large use of this technique could generate billions of dollars and thousands of jobs all while saving the environment. The process used is fairly simple. They take mixed plastic polymers without much sorting, convert them to a liquid hydrocarbon in an oxygen free environment, and then refine the hydrocarbon into diesel and fuel. The two major industry leaders are located in Ohio and London. They are rapidly expanding and developing new technologies. The article states that "when operating at full capacity each unit handles 60 tons of plastic per day, which equates to 1.4 million liters per year of transportation fuel." The technology is up and running it is just going to take some time to get it to the market, but it is good to see this industry taking an initiative towards a cleaner environment. For more info, click here for one of the company's website.
Subscribe to:
Comments (Atom)